Epilepsy is one of the
most prevalent neurological condition characterized by spontaneously
non-provoked seizures that occur as a result of a complex disorder of network
homeostasis. Current treatments focus on the suppression of these epileptic
seizures. However, research has come across evidence for the prevention of
epileptogenesis (development and progression of epilepsy) through biochemical
manipulations. Detlev Boison, in his review, The Biochemistry and Epigenetics of Epilepsy: Focus on Adenosine and
Glycine has discussed this concept via the mechanisms implicated in
epileptogenesis and the biochemical interactions between adenosine and glycine which
serve as mayor contributors to the development of epilepsy.
Boisin focuses on
temporal lobe epilepsy (TLE), especially its key metabolites adenosine and
glycine. These are primitive biological
elements with important biochemical functions, whose homeostasis is generally affected
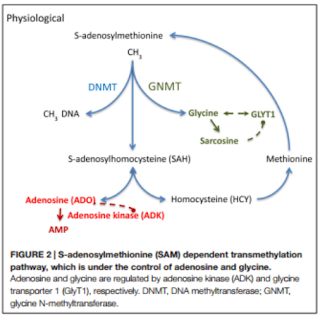
in epileptic brains. Adenosine is an endogenous anticonvulsant and seizure
terminator only when the adenosine A1 receptors is activated. Overexpression of
its kinase (ADK), results in an adenosine deficiency associated with the increase
of astrocytes, known as astrogliosis, and the adenosine receptor (AR) has been
linked to the control of DNA methylation under the activity of ADK. The latter
is expressed in both cytoplasmic and nuclear isoforms whose functions range
from homeostatic regulation to modification in DNA methylation statuses. Astrogliosis
is closely related with the increase in ADK expression and the deficiency of
adenosine, which leads to the production of seizures and the hypermethylation
of DNA. Therefore, it can be concluded that the dysregulation of ADK has a
significant effect in the process of turning a normal brain into an epileptic
one. Glycine, on the other hand, may have various effects depending on the
activation of its presynaptic or postsynaptic receptor (GlyR’s). Low concentrations
of glycine have pro-convulsive effects, while high concentrations reduce its occurrence.
Its homeostasis is crucial in maintaining a balance in neuronal excitability
and its regulation and reuptake is achieved by the glycine transporter 1 (GlyT1).
The latter, when increased, is associated with TLE and has been considered a
promising target for treatments of cognitive diseases.
“The knowledge of
epigenetic mechanisms implicated in the development of epilepsy provides a
conceptual and mechanistic framework for the future development of epigenetic
therapies tailored to prevent epilepsy (antiepileptogenic) or its progression
(disease modifying)”(Boison, 2016). The current treatments fail to take into
account the causes of epilepsy and are therefore unable to halt
epileptogenesis, which is why epigenetic modifications offer new therapeutic
alternatives. Using rat models it has been discovered that an adenosine
augmentation can effectively reduce and even suppress the occurrence of
seizures. They have also been used to form the basis for both the ADK hypothesis
(acute insults to the brain such as traumatic brain injury, seizures, or a
stroke lead to an acute surge in adenosine associated with transient downregulation
of ADK) and the methylation hypothesis of epileptogenesis (suggests that
seizures may induce epigenetic modifications aggravating the condition). Alteration
in DNA methylation plays a significant role in in the development and
progression of neurodegenerative diseases like epilepsy. The increased activity
of DNA methylating enzymes and the hypermethylation of DNA has been linked to
onset of epilepsy. However, the status of DNA methylation depends on the
equilibrium of biochemical enzyme reactions catalyzed by DNA methyltransferases
(DNMTs) or Ten-eleven translocations (TET) enzymes. These mechanisms depend on transmethylation
pathways controlled by adenosine and glycine concentrations regulated by ADK
and GlyT1.
The biochemical
discoveries discussed by Boison have made way for new research areas.
Understanding the epigenetics behind epilepsy may be result in the development
of novel and effective therapeutic strategies.
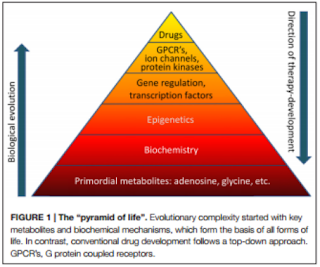
“Challenges
for drug development remain. It needs to be determined whether new therapeutic
agents can enter the brain and whether a higher level of selectivity for
specific isoforms of ADK can be achieved. Due to the different distribution of
nucleoside transporters within the brain there might be opportunities for the
development of cell-type or isoform selective therapies.” (Boison, 2016).
References:
Boison, D. (2016). The Biochemistry and Epigenetics of
Epilepsy: Focus on Adenosine and Glycine. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4829603/pdf/fnmol-09-00026.pdf
¿Han hecho pruebas en ratas provocando un aumento en glicina, como lo han hecho con la adenosina, para ver si logran suprimir las convulsiones?
ReplyDeleteEl artículo nos menciona la frase "DNA methylation plays a significant role in in the development and progression of neurodegenerative diseases like epilepsy". ¿Qué otras enfermedades también son causadas por la metilación de ADN? ¿Todas son espontáneas al igual que la epilepsia?
ReplyDeleteSaludos Danny.
DeleteEfectivamente, existen otras enfermedades en el que la metilación del ADN juega un papel esencial. Entre estas se encuentran Alzheimer's disease, Parkinson's disease, Huntington's disease, y amyotrophic lateral sclerosis (ALS). Pueden ocurrir de manera espontánea en un individuo, o ser causadas o influenciadas por la historia familiar del individuo.
En la cita que mencionan al final, la epigenética abre nuevos retos por descubrir en el mundo del desarrollo de las drogas y la farmacología. Creen que entonces sea posible el surgimiento de "drogas o terapias epigenéticas" para combatir enfermedades afectadas por mecanismos epigenéticos?
ReplyDeleteSaludos Nicole.
Delete¡Efectivamente! Gran parte del interés en la epigenética es debido a su relación con enfermedades, y por consiguiente, en la posibilidad de desarrollar nuevos métodos para predecir, diagnosticasr, tratar, e incluso curar enfermedades.
Uno de retos que se presentan en el campo de la farmacología para el tratamiento de tumores cerebrales es el diseño de drogas específicas de tamaños bien pequeños que logren atravesar el "blood brain barrier", Creen que estas drogas serían la solución a enfermedades como epilepsia y que al mismo tiempo no induzcan otros mecanismos epigenéticos o toxicológicos?
ReplyDeleteSegún la opinioón de ustedes, cuál creen es la mayor aportación que podría hacer el estudio y entendimiento de la epigenética?
ReplyDeleteSaludos Nicole. En mi opinión, por encima de todos los beneficios y conocimientos que nos brinda el estudio del campo de la epigenética, la mayor aportación que puede brindar es en ayudar a entender y tratar enfermedades. La posibilidad de sencuenciar el ADN del paciente (que se vuelve cada vez más accesible), y realizar otras pruebas para establecer cuáles son las marcas epigenéticas presentes podría dar paso a el desarrollo de tratamientos más personalizados para cada individuo.
DeleteIs it safe to say that increasing or decreasing the concentration on both Adenosine and Glycine has the same effect, since low concentrations of both tend to be related with seizures? Or is the effect more significant in altering the concentration of one over the other?
ReplyDelete