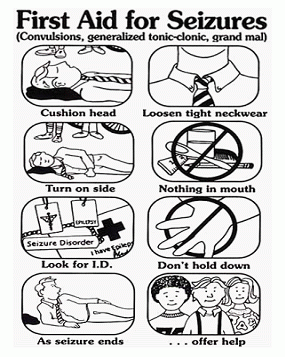
Welcome to the beautiful and mysterious world of Epigenetics! This blog will lead the way in a step by step comprehension, towards this unusually heard of- field of science. The involvement of epigenetics in neurodegenerative disorders, such as epilepsy, is of vital importance.
Tuesday, November 15, 2016
Epigenetic Mechanisms in Stroke and Epilepsy
DNA and histone methylation cause epigenetic remodeling which represents central mechanisms for the regulation of neuronal gene expression during brain development, higher-order processing and memory formation. Recent studies have discovered that chromatin modifications have significant roles in neurodegenerative diseases associated with ischemic stroke and epilepsy via the activation of REST, a gene silencing transcription factor which leads to epigenetic remodeling of transcriptionally responsive targets implicated in neuronal death.
References:
Hwang, J., Aromolaran, K., Zukin, R.S. (2013). Epigenetic Mechanisms in Stroke and Epilepsy.
Retrieved from http://www.nature.com/npp/journal/v38/n1/pdf/npp2012134a.pdf
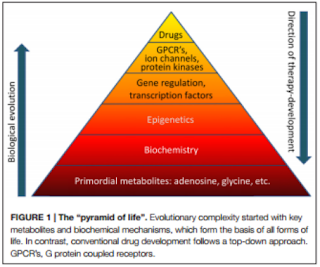
The Biochemistry and Epigenetics of Epilepsy: Focus on Adenosine and Glycine
Epilepsy is one of the
most prevalent neurological condition characterized by spontaneously
non-provoked seizures that occur as a result of a complex disorder of network
homeostasis. Current treatments focus on the suppression of these epileptic
seizures. However, research has come across evidence for the prevention of
epileptogenesis (development and progression of epilepsy) through biochemical
manipulations. Detlev Boison, in his review, The Biochemistry and Epigenetics of Epilepsy: Focus on Adenosine and
Glycine has discussed this concept via the mechanisms implicated in
epileptogenesis and the biochemical interactions between adenosine and glycine which
serve as mayor contributors to the development of epilepsy.
Boisin focuses on
temporal lobe epilepsy (TLE), especially its key metabolites adenosine and
glycine. These are primitive biological
elements with important biochemical functions, whose homeostasis is generally affected
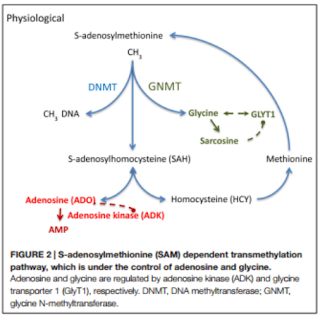
in epileptic brains. Adenosine is an endogenous anticonvulsant and seizure
terminator only when the adenosine A1 receptors is activated. Overexpression of
its kinase (ADK), results in an adenosine deficiency associated with the increase
of astrocytes, known as astrogliosis, and the adenosine receptor (AR) has been
linked to the control of DNA methylation under the activity of ADK. The latter
is expressed in both cytoplasmic and nuclear isoforms whose functions range
from homeostatic regulation to modification in DNA methylation statuses. Astrogliosis
is closely related with the increase in ADK expression and the deficiency of
adenosine, which leads to the production of seizures and the hypermethylation
of DNA. Therefore, it can be concluded that the dysregulation of ADK has a
significant effect in the process of turning a normal brain into an epileptic
one. Glycine, on the other hand, may have various effects depending on the
activation of its presynaptic or postsynaptic receptor (GlyR’s). Low concentrations
of glycine have pro-convulsive effects, while high concentrations reduce its occurrence.
Its homeostasis is crucial in maintaining a balance in neuronal excitability
and its regulation and reuptake is achieved by the glycine transporter 1 (GlyT1).
The latter, when increased, is associated with TLE and has been considered a
promising target for treatments of cognitive diseases.
“The knowledge of
epigenetic mechanisms implicated in the development of epilepsy provides a
conceptual and mechanistic framework for the future development of epigenetic
therapies tailored to prevent epilepsy (antiepileptogenic) or its progression
(disease modifying)”(Boison, 2016). The current treatments fail to take into
account the causes of epilepsy and are therefore unable to halt
epileptogenesis, which is why epigenetic modifications offer new therapeutic
alternatives. Using rat models it has been discovered that an adenosine
augmentation can effectively reduce and even suppress the occurrence of
seizures. They have also been used to form the basis for both the ADK hypothesis
(acute insults to the brain such as traumatic brain injury, seizures, or a
stroke lead to an acute surge in adenosine associated with transient downregulation
of ADK) and the methylation hypothesis of epileptogenesis (suggests that
seizures may induce epigenetic modifications aggravating the condition). Alteration
in DNA methylation plays a significant role in in the development and
progression of neurodegenerative diseases like epilepsy. The increased activity
of DNA methylating enzymes and the hypermethylation of DNA has been linked to
onset of epilepsy. However, the status of DNA methylation depends on the
equilibrium of biochemical enzyme reactions catalyzed by DNA methyltransferases
(DNMTs) or Ten-eleven translocations (TET) enzymes. These mechanisms depend on transmethylation
pathways controlled by adenosine and glycine concentrations regulated by ADK
and GlyT1.
The biochemical
discoveries discussed by Boison have made way for new research areas.
Understanding the epigenetics behind epilepsy may be result in the development
of novel and effective therapeutic strategies.
“Challenges
for drug development remain. It needs to be determined whether new therapeutic
agents can enter the brain and whether a higher level of selectivity for
specific isoforms of ADK can be achieved. Due to the different distribution of
nucleoside transporters within the brain there might be opportunities for the
development of cell-type or isoform selective therapies.” (Boison, 2016).
References:
Boison, D. (2016). The Biochemistry and Epigenetics of
Epilepsy: Focus on Adenosine and Glycine. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4829603/pdf/fnmol-09-00026.pdf
Saturday, November 12, 2016
Diving deeper: some epigenetic changes in epilepsy
The establishment and maintenance of epigenetic marks are crucial for normal development and function. Here, we will discuss how DNA methylation patterns are altered, and the presence od certain histone variants and microRNA's is changed in epilepsy.
DNA Methylation
There is evidence in recent studies in animal models and tissues from epileptic patients that have revealed altered methylation patterns in DNA in these patients, compared to healthy individuals.
An autopsy study of the hippocampus tissue from patients with temporal lobe epilepsy found a greater degree of methylation of the reelin promoter among the patients with epilepsy compared to controls (Kobow et al., 2009). Reelin is an extracellular matrix protein that performs key functions in neuronal migration, synaptic plasticity, and maintenance of the laminar structure of hippocampal granule cells. Loss of this structure in the hippocampal dentate nucleus (granule dispersion) is present in up to 50% of the patients affected by temporal lobe sclerosis.
Additionally, another study examined DNMT1 and DNMT3A (DNA methyltransferase 1 and 3A) expression in patients with temporal lobe epilepsy compared to healthy controls. Remember that DNMT1 is responsible for the maintenance of methylation patterns, and DNMT3a and DNMT3b were involved in de novo methylations. The study concluded that both DNMT’s are more abundant in patients with temporal lobe epilepsy, hinting that they may contribute to the pathogenesis of this type of epilepsy (Zhu et al., 2011).
Finally, a study analyzed the overall DNA methylation in the hippocampus of rats with chronic epilepsy compared with controls. The group with chronic epilepsy showed more overall methylation (Kobow et al., 2013). But when provided with a ketogenic diet, they observed a reduction in seizure frequency and a change in the DNA methylation pattern.
Histone modification
Studies in animal models have demonstrated changes in chromatin mediated by histone modifications following epileptic seizures.
For example, a study analyzed rat hippocampal tissue 3 hours after having induced status epilepticus and found that histone H4 hypoacetylation (which is a marker for gene repression) in the promoter of the glutamate 2 receptor (GluR2), as well as hyperacetylation (a marker for gene transcription) in the brain-derived neurotrophic factor (BDNF) promoter (Huang et al., 2002). These findings clearly show that status epilepticus* rapidly triggers modulations in histone acetylation. The same study found that prior administration of an HDAC (histone deacetylase) inhibitor prevented hyperacetylation of the GluR2 promoter, which could help design a treatment for epilepsy.
In a more recent study, the same author reported greater HDAC2 expression in tissue from patients with temporal lobe epilepsy, as well as from animal subjects with status epilepticus, than in controls (Huang et al., 2011). HDAC2 is a type of HDAC expressed by the central nervous system that is active in neurodevelopment. Results from the study show that HDAC2 is significantly involved in the pathogenesis of temporal lobe epilepsy, and in the cognitive impairment that may sometimes also be associated with this type of epilepsy.
In another animal model of epilepsy using electrically induced seizures, Tsankova et al. found changes in the acetylation of histones H3 and H4 at the CREB promote region in the rat hippocampus, with H4 hypoacetylation of CREB and H3 hyperacetylation of CREB miRNA (Tsankova et al., 2004). CREB is an important transcriptional factor that plays an important role in the epileptogenic process.
Micro-RNA and epilepsy
Several studies of the expression profile of miRNA in epilepsy have been published recently, and they offer promising information about the potential role of miRNA as a biomarker.
One example is a study that describes the miRNA expression profile in rats with induced status epilepticus based on analyses of brain tissue and blood samples (Liu et al., 2009). The authors found similar expression profiles for one miRNA subtype in blood and hippocampal tissue and therefore support the possibility that miRNA's might serve as blood biomarkers for epilepsy.
On the other hand, studies of miRNA are also providing additional knowledge about the epileptogenic process.
For example, various studies carried out in animal models have all shown increased expression of miRNA-132 in the hippocampus of rats with induced status epilepticus (Pulido et al., 2015). It is understood that miRNA-132 has anti-inflammatory functions, and inflammation has been shown to play a role in epileptogenesis. Therefore, increased miRNA-132 may contribute to the development of epilepsy.
*Status epilepticus is said to occur when a seizure lasts too long or when seizures occur close together and the person doesn't recover between seizures.
More information can be found in this paper:
Pulido Fontes, L., Quesada Jimenez, P., & Mendioroz Iriarte, M. (2015). Epigenetics and epilepsy. Neurología (English Edition), 30(2), 111-118. http://dx.doi.org/10.1016/j.nrleng.2014.03.002
Wednesday, November 2, 2016
Basic epilepsy knowledge
What is epilepsy?
Epilepsy is a central nervous system disorder (neurological disorder) in which nerve cell activity in the brain becomes disrupted, causing seizures or periods of unusual behavior, sensations and sometimes loss of consciousness (Mayo Clinic). Seizure symptoms can vary widely, from low to high intensity. Difficulty breathing and temporary confusion, are also symptoms related to epilepsy.
Who gets affected by epilepsy and what are the causes?
People of any age can get affected by epilepsy, especially if there is any structural brain lesion. It is, however, more common in young children and older people. The disorder can be developed through life or be present since birth. Car accidents, falling, gun shots, pregnancy complications and emotional issues can affect or cause epilepsy. Additional factors such as health conditions, age, and race can make its development more likely. For example, it is more common in people with Hispanic backgrounds.
How is epigenetics involved in epilepsy?
The methylation hypothesis of epileptogenesis (development and progression of epilepsy) suggests that changes in DNA methylation are implicated in the progression of the disease. In particular, global DNA hypermethylation appears to be associated with chronic epilepsy (Boison, 2016).
Can it be treated or cured?
Currently, there are no cures for epilepsy, but instead the seizures and symptoms are controlled by specific medications. Fortunately, epigenetic influences in epilepsy are being studied, and since epigenetic changes are reversible, there may be an answer in the near future.
For more information,
http://perspectivesinmedicine.cshlp.org/content/5/12/a022731.long
Epilepsy is a central nervous system disorder (neurological disorder) in which nerve cell activity in the brain becomes disrupted, causing seizures or periods of unusual behavior, sensations and sometimes loss of consciousness (Mayo Clinic). Seizure symptoms can vary widely, from low to high intensity. Difficulty breathing and temporary confusion, are also symptoms related to epilepsy.
Who gets affected by epilepsy and what are the causes?
People of any age can get affected by epilepsy, especially if there is any structural brain lesion. It is, however, more common in young children and older people. The disorder can be developed through life or be present since birth. Car accidents, falling, gun shots, pregnancy complications and emotional issues can affect or cause epilepsy. Additional factors such as health conditions, age, and race can make its development more likely. For example, it is more common in people with Hispanic backgrounds.
How is epigenetics involved in epilepsy?
The methylation hypothesis of epileptogenesis (development and progression of epilepsy) suggests that changes in DNA methylation are implicated in the progression of the disease. In particular, global DNA hypermethylation appears to be associated with chronic epilepsy (Boison, 2016).
Can it be treated or cured?
Currently, there are no cures for epilepsy, but instead the seizures and symptoms are controlled by specific medications. Fortunately, epigenetic influences in epilepsy are being studied, and since epigenetic changes are reversible, there may be an answer in the near future.
For more information,
http://perspectivesinmedicine.cshlp.org/content/5/12/a022731.long
Subscribe to:
Comments (Atom)