When referring to epilepsy, we are referring to
a heterogeneous group of disorders, including both those of genetic origin and
those acquired. They are associated with a number of pathogenic mechanisms,
seizure manifestations, comorbidity profiles and therapeutic responses. It has
been observed through clinical and translation research that many of these
epileptic features are closely affect by sex differences, especially hormones. That
is, there is emerging evidence that common pathological features in epilepsy
syndrome are linked with sex differences with males exhibiting a greater
incidence than females. Through this paper, Qureshi et al, explain the primary
epigenetic mechanisms and how these are now being used to integrate hormonal
and genetic influences at molecular, cellular and network levels in epileptic
disorders and the process of epilptogenesis (described in previous post).
“The
foremost epigenetic mechanisms include DNA methylation (and
hydroxymethylation), histone protein post-translational modifications (PTMs)
and higher-order chromatin remodeling, and noncoding RNA (ncRNA) regulation.
These multilayered processes are highly interconnected and exert their
regulatory effects through coordinate actions.”
Epigenetic mechanisms are mediator of the brains form and function and
it believed to be a source in the promotion of dimorphism in the brain and
body. They help establish and maintain sex differences in gene expression, for
example the X inactivation-specific transcript (XIST) and genomic imprinting
(more details related to its function in paper). Various epigenetic factors are
expressed in sex-specific patterns in the breain known to be dimorphic, however
these factors and there mechanisms are sensitive to sex steroid hormone
pathways and exposure. Sex modulation has also been observed in autosomally
encoded factors. “These observations suggest that epigenetic regulators in
brain are deployed in a sex-specific manner, consistent with other evidence
from expression quantitative trait loci analyses revealing sex-biased gene
regulatory architectures in human brain.”
Qureshi et al. describe various non-mutually exclusive paradigms
relating epigenetic factors and neurological diseases including: : mutations in
genes encoding epigenetic factors that cause disease, genetic variation in
genes encoding epigenetic factors modifying disease risk, and the expression
and function of epigenetic factors targeting disease-associated genomic loci,
gene products, and cellular pathways. The first has proven to be true linking
DNA methylation and histone modifying enzymes with the onset of the disease. Emerging
data on the second paradigm has demonstrated variability in the vulnerability
to epileptic disorders, like for example polymorphisms of the
bromodomain-containing protein 2 (BRD2) gene which confers susceptibility to
common forms of myoclonic epilepsy. This has also been studied in mice,
demonstrating a sex-specific decrease in seizure thresholds. Lastly, evidence indicating
that an increase in DNA methylation in the hippocampus are associated with
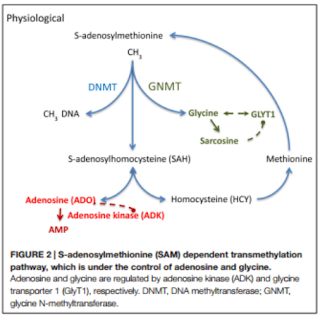
epileptogenesis and that adenosine exhibit inhibition of DNA methylation (described in previous post), supports
the third paradigm.
The observations detailed throughout the paper suggest that the epigenetic
factors and mechanisms in males and females underlie sex differences associated
with risk, onset, and progression of epileptic disorders. However, seeing as
many bodily functions work in coaction it is important to study other pathways
and how these interact with both epigenetic factors and epilepsy. In this way,
it might be possible to generate a novel therapeutic drug to treat these and
other diseases more efficiently.
Reference: Qureshi, I.A., Mehler, M., (July 4, 2014). Sex, Epilepsy and Epigenetics. Elsevier, Retrieved from http://ac.els-cdn.com/S0969996114001831/1-s2.0-S0969996114001831-main.pdf?_tid=7ae5413c-bdc7-11e6-a331-00000aacb361&acdnat=1481257694_2ef9836c0fdfbf1053537c6b34baff56